
Many of the advances made in chemistry during the 18th and 19th centuries were the result of careful experiments done to determine the identity and quantity of gases produced in chemical reactions. How much iron (in kilograms) was needed to produce this volume of H 2 if the temperature was 30☌ and the atmospheric pressure was 745 mmHg?Īnswer: 68.6 kg of Fe (approximately 150 lb) The hydrogen gas was produced by the reaction of metallic iron with dilute hydrochloric acid according to the following balanced chemical equation: Fe(s) + 2 HCl(aq) → H 2(g) + FeCl 2(aq) In Example 5, we saw that Charles used a balloon containing approximately 31,150 L of H 2 for his initial flight in 1783. These numbers may give you some appreciation for the magnitude of the engineering and plumbing problems faced in industrial chemistry. The answer means that more than 300,000 L of oxygen gas are needed to produce 1 tn of sulfuric acid. We next calculate the number of moles of O 2 required: moles O 2 = ( 9250 mol H 2 SO 4 ) ( 1.5 mol O 2 1 mol H 2 SO 4 ) = 1.39 × 10 4 mol O 2ī After converting all quantities to the appropriate units, we can use the ideal gas law to calculate the volume of O 2: T = 273 + 22 = 295 K P = ( 745 mmHg ) ( 1 atm 760 mmHg ) = 0.980 atm Volume of O 2 = n O 2 ( R T P ) = ( 1.39 × 10 4 mol O 2 ) [ 0.08206 (L We proceed exactly as in Chapter 3 "Chemical Reactions", using the strategy mass of H 2SO 4 → moles H 2SO 4 → moles O 2 → liters O 2Ī We begin by calculating the number of moles of H 2SO 4 in 1.00 tn: moles H 2 SO 4 = ( 1.00 tn H 2 SO 4 ) ( 2000 lb 1 tn ) ( 453.6 g 1 lb ) ( 1 mol H 2 SO 4 98.08 g ) = 9250 mol H 2 SO 4
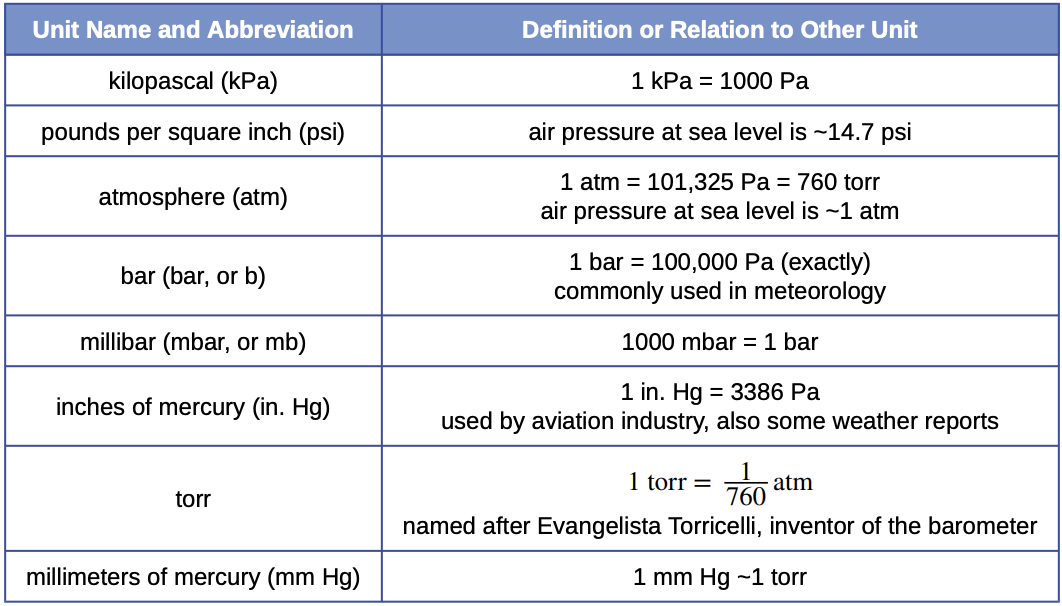

This is a standard stoichiometry problem of the type presented in Chapter 3 "Chemical Reactions", except this problem asks for the volume of one of the reactants (O 2) rather than its mass. We can see from the stoichiometry of the reaction that 3 2 mol of O 2 is required to produce 1 mol of H 2SO 4. Be sure that all quantities are expressed in the appropriate units. From the stoichiometric coefficients in the balanced chemical equation, calculate the number of moles of O 2 required.ī Use the ideal gas law to determine the volume of O 2 required under the given conditions. Given: reaction, temperature, pressure, and mass of one productĪ Calculate the number of moles of H 2SO 4 in 1.00 ton. What volume of O 2 (in liters) at 22☌ and 745 mmHg pressure is required to produce 1.00 ton of H 2SO 4? The overall chemical equation is as follows: S + 3 2 O 2 + H 2 O → H 2 SO 4 Sulfuric acid, the industrial chemical produced in greatest quantity (almost 45 million tons per year in the United States alone), is prepared by the combustion of sulfur in air to give SO 2, followed by the reaction of SO 2 with O 2 in the presence of a catalyst to give SO 3, which reacts with water to give H 2SO 4.

As a chemical engineer said to one of the authors, “Gases always go where you want them to, liquids sometimes do, but solids almost never do.” Gases mix readily, are easily heated or cooled, and can be transferred from one place to another in a manufacturing facility via simple pumps and plumbing. Furthermore, many, if not most, industrially important reactions are carried out in the gas phase for practical reasons. Many reactions that are carried out in the laboratory involve the formation or reaction of a gas, so chemists must be able to quantitatively treat gaseous products and reactants as readily as they quantitatively treat solids or solutions. With the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to calculate the stoichiometry of reactions involving gases, if the pressure and temperature are known.


 0 kommentar(er)
0 kommentar(er)
